Porex Provides Key Component in COVID-19 Tests
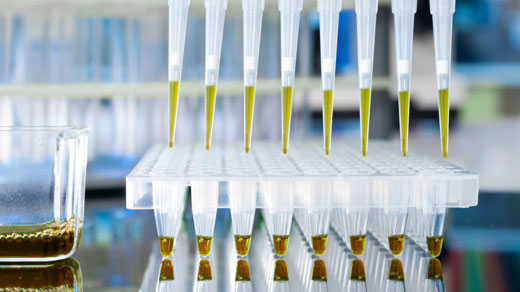
The new COVID-19 tests – approved under the FDA’s Emergency Use Authorization – utilize a type of technology that measures RNA rather than DNA. Only filtered or aerosol barrier pipette tips are mandated for use in order to reduce the risk of aerosol-related contamination. Porex has been manufacturing filters for aerosol barrier pipette tips for over 25 years and is the leading global supplier.
Porex recently received a large pipette tip filter order from a large diagnostic instrument manufacturer for their COVID-19 testing system, the first commercially developed test to be approved by the FDA for the disease. Other diagnostic instrument manufacturers have since been approved by the FDA to produce similar tests and will also use Porex pipette tip filters. #MADISONSTRONG

